Laparoscopic partial nephrectomy in warm ischemia: impact of parenchymal loss on kidney function
DOI: 10.22591/magyurol.2017.4.bagherif.158
Authors:
Fariborz Bagheri dr.1,2, Csaba Pusztai dr.1, László Farkas dr.1, István Buzogány dr.3, Zsuzsanna Szabó dr.4, János Lantos dr.5, Marianna Imre dr.6, Nelli Farkas dr.7, Árpád Szántó dr.1
1University of Pécs Medical School, Department of Urology, Pécs, Hungary (director: Árpád Szántó dr.)
2Dubai Health Authority, Dubai Hospital, Department of Urology, Dubai, UAE
3Péterfy Sándor Street Hospital, Department of Urology, Budapest, Hungary (director: István Buzogány dr.)
4University of Pécs Medical School, Department of Nuclear Medicine, Pécs, Hungary (director: Katalin Zámbó dr.)
5University of Pécs Medical School, Department of Research and Techniques, Pécs, Hungary (director: Gábor Jancsó dr.)
6Diagnostic Center of Pécs, Hungary (director: Ferenc Kövér dr.)
7University of Pécs Medical School, Institute of Bioanalysis, Pécs, Hungary (director: Ferenc Kilár dr.)
Summary
Objective: To evaluate impact of deliberate renal parenchymal loss on renal function, distinguished from ischemic reperfusion (I-R) injury after laparoscopic partial nephrectomy (LPN) under warm ischemia (WI).
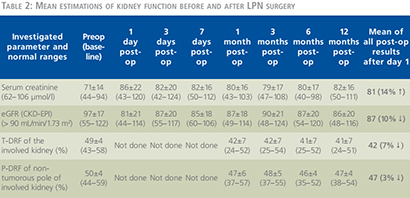
Patients and methods: Thirty five patients with a single polar renal mass £4 cm with normal contralateral kidney planned for transperitoneal laparoscopic partial nephrectomy (LPN) under WI using en-bloc hilar occlusion in a prospective study. Total differential renal function (T-DRF) using 99TTc-Dimercaptosuccinic acid (99TTc-DMSA) was evaluated preoperatively and postoperatively in different intervals over one year. In all isotope assessments, a special region of interest (ROI) was selected on the non-tumorous pole of the involved kidney and it was compared with the same ROI in the contralateral kidney. This was named as partial DRF (P-DRF). Any postoperative decline in the P-DRF of the operated kidney was contributed to I-R injury unrelated to parenchymal loss. Subtraction of the P-DRF decline from the T-DRF decline was attributed to the parenchymal loss caused by deliberate resection and suturing of the normal parenchyma. Other than DRF, serum creatinine (sCr), estimated glomerular filtration rate (eGFR) using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, warm ischemia time (WIT), and weight of the resected specimen were also measured.
Results: The mean WIT was 22 minutes, and the mean weight of resected specimen was 18 grams. The mean postoperative eGFR declined to 87 from its baseline mean value of 97 (p-value = 0.0757). Mean postoperative T-DRF and P-DRF of the operated kidney, declined by 7% and 3% respectively.
Conclusion: After LPN of small renal mass, decline in renal function is primarily contributed to parenchymal loss caused by deliberate resection and suturing of the normal parenchyma rather than the I-R injury.
LAPSZÁM: MAGYAR UROLÓGIA | 2017 | 29. ÉVFOLYAM, 4. SZÁM
Összefoglalás
Célkituzés: A veseparenchyma-veszteség hatásának meghatározása a vesefunkcióra elkülönítve a reperfúzió károsodástól (I-R) meleg iszkémiában végzett parciális nephrectomia során.
Betegek és módszer: Tanulmányukba harmincöt, £4 cm vesedaganat miatt meleg iszkémiában végzett transzperitoneális laparoszkópos parciális nephrectomián átesett, ellenoldali ép vesével rendelkezo beteg került prospektíven beválasztásra. Meghatároztuk az egyes vesék funkcióját 99mTc-Dimercaptosuccinic acid (99TmTc-DMSA) veseszcintigráfiával a mutét elott, posztoperatíve és a nyomonkövetés során meghatározott idopontokban egy évig (Total differential renal function – T-DRF). Összehasonlítottuk a daganatos vese nem daganatos pólusának és az ellenoldali vese azonos lokalizációjú területének funkcióját ROI (region of interest) technikával (partial DRF – P-DRF). Az operált és nem operált vesék nem daganatos területeinek funkciójában észlelt csökkenés (P-DRF) a reperfúzió (I-R) hatásának volt betudható. A P-DRF és a T-DRT-csökkenés különbsége parenchyma-veszteségnek tulajdonítható, amelyet a parenchyma-reszekció és varrat okozott. Ezen kívül vizsgáltuk a Se kreatinin értékek, a glomeruláris filtrációs ráta (eGFR) változását, a meleg iszkémiás idot (WIT) és az eltávolított veseszövet súlyát.
Eredmények: Az átlagos meleg iszkémiás ido 22 perc, az eltávolított veseszövet súlya 18 g volt. A posztoperatív eGFR-értéke átlagosan 87 ml/perc volt a kiindulási átlagos 97 ml/perchez képest (p-value = 0,0757). A posztoperatív T-DRF és P-DRF csökkenés átlaga 7%, illetve 3% volt.
Megbeszélés: A laparoszkópos parciális nephrectomiát követoen észlelt vesefunkció-csökkenés oka elsodlegesen a veseparenchyma-veszteség és varrat, az I-R szerepe lényegesen kisebb.
Introduction
Partial nephrectomy (PN) has become a standard of care for treatment of small renal masses. Hilar occlusion is commonly performed for a precise tumour resection and renal reconstruction. The above surgical manoeuvre results in warm ischemia (WI) of the remaining renal tissue and has been associated with ischemic-reperfusion injury (RI) to the organ. Current evidence showed that the length of the warm ischemia time (WIT) and the subsequent reperfusion injury may result in permanent renal damage (1, 2). Moreover, the resection of the renal tumour and the suturing of the parenchyma resulted in additional reduction of the functional renal tissue (3, 4). Thus, two mechanisms of renal function damage during PN could be proposed. Nevertheless, the importance of the mechanisms for the decline of the postoperative renal function has not been investigated. The current prospective study evaluated the split renal function and elucidated the role of renal parenchymal loss in patients with small renal mass who were treated by LPN with WI.
Patients and methods
Small renal masses have been treated by LPN at our institutions since 2005. Thirty five patients were enrolled in a prospective pilot study. Regional research ethics committee approval was received and informed consent was obtained from all patients. The procedures were performed by two experienced laparoscopists. The exact location and dimensions of the tumour was identified by three-dimensional CT scan prior to the operation. Only patients with a single exophytic mass of £4 cm in diameter located in either lower or upper pole of the kidney with normal contralateral kidney were enrolled.
All operations were performed by laparoscopic transperitoneal approach with en-bloc hilar occlusion using a Rumel tourniquet. Two minutes before hilar occlusion, 0.5 gr/kg of 20% mannitol was infused. The surgical technique has been previously described (5). Cold scissors were used for tumour resection. Running sutures of 3/0 Vicryl were applied for collecting system closure and haemostasis. In all cases, the parenchymal defect was filled with one or two rolled Surgical bolsters. Parenchymal reconstruction was achieved by running 0 Vicryl sutures secured at each parenchymal exit by a Hem-o-lok clip. The renal pedicle was released only after tumour excision and completion of renorrhaphy. At the time of hilar tourniquet release, 0.5 mg/kg of Furosemide injection was administered. The kidney was placed in its anatomic position and the Gerota’s fascia was closed.
After extraction of the specimen, the surrounding fat tissues were detached and weight of the remaining resected mass was measured. The recorded parameters included the time for tumour resection, calyceal closure, haemostatic sutures and the total WIT.
Serum creatinine (sCr) was recorded and estimated glomerular filtration rate (eGFR) was calculated using chronic kidney disease epidemiology collaboration (CKD-EPI) equation (6).
The above measurements were performed preoperatively (baseline), 5-6 hours after the surgery, on the 1st, 3rd and 7th postoperative days and at the end of 1st, 3rd, 6th and 12th postoperative months.
In this study, in addition, 15 randomized patients treated with laparoscopic radical nephrectomy (LRN) were examined as a control group for our laboratory outcomes and all the above parameters were registered in this group.
Renal scintigraphy
In order to distinguish the impact of parenchymal loss from WI effect on the operated kidney, we planned a novel method of investigation with renal scan as follow. All patients in LPN group underwent 99m Technetium-Dimercaptosuccinic Acid (99mTc-DMSA) renal scintigraphy for the determination of split renal function preoperatively and at the end of 1st, 3rd, 6th and 12th postoperative months.
99mTc-DMSA isotope is a static renal agent and allows accurate calculation of differential renal function (DRF) (7). Since 99mTc-DMSA scan provides relative functional percentage of the two kidneys and the contralateral kidney served as a control for comparison after LPN, we selected patients with solitary small polar mass (T1a), otherwise normal ipsilateral kidney, and normal contralateral kidney. Such selections have resulted in a young cohort of patients with mean age of 50.5±11.9 years old in our study.
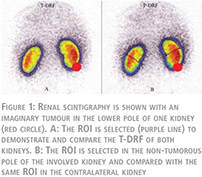
Assessment of total and partial differential renal function
A novel approach
Before the operation, all patients underwent radionuclide isotope examination performed by 99mTc-DMSA. Renal scans were performed in supine position. Individual kidney uptake and differential renal function (DRF) percentage of left-to-right kidneys were determined by the Patlak-Rutland method (8). The region of interest (ROI) of each kidney was determined with the use of an automated computer program drawing the ROI around the whole kidney. For processing purposes, all isotope results were saved in a computer program. This assessment which is a customary method of evaluating a static renal scan was called a “Total-DRF” (T-DRF) in our study (Fig. 1A).
By this means, in the tumorous kidney, the postoperative decline in percentage ratio, reflecting decrease in renal function, was considered as a consequence of both factors:
- IR injury caused by length of WIT;
- The kidney parenchymal volume reduction caused by removal of the tumour, and excision and suturing of the surrounding healthy tissues.
In an attempt to distinguish the impact of each of these two factors, we introduced a novel method which is referred as “Partial-DRF” (P-DRF). For this reason, in the preoperative renal scans, the exact location of the tumour was determined and only small polar masses (either upper or lower pole mass) were selected for the study. In the tumorous kidney, a region in the tumour-free pole was selected and manually a ROI was drawn in that pole. Identical ROI was selected in the same pole of the contralateral kidney (Fig. 1B). The same ROI drawing was used in all follow-up studies of a given patient. Accordingly, P-DRF which reflects DRF of the intact pole of the operated kidney, which is affected only by the IR injury, was compared with the same pole on the contralateral kidney. The same processing was applied for all patients in all isotope scan examinations. As a result, in the postoperative isotope scans, with the P-DRF, we could compare an intact part of the operated kidney which was impacted by WI but not affected by parenchymal volume reduction with an identical segment of the normal contralateral kidney.
Any postoperative decline in the P-DRF of the operated kidney was considered as the renal functional loss resulted from IR injury only.
All renal isotope tests were evaluated and reported by same specialist doctor in nuclear medicine.
Patient selection and statistical evaluations
Any factor which could unpredictably influence on the WI consequence or the renal function outcome was excluded from the statistical assessments. Accordingly, one patient was excluded due to conversion to open partial nephrectomy. One patient was excluded due to continuous moderate bleeding from the resected site during WI time. In one patient with two accessory renal arteries, we had to clip one of the arteries for safe resection. In one case, five days after the operation, selective segmental arterial embolization of the operated kidney was applied due to arteriovenous shunt. Three patients were disqualified for statistical analysis since they missed more than one cycle of post-operative follow-ups. Consequently, 28 patients were enrolled in the final statistical analysis. Demographics of the 28 patients and results of the operations are described in Table 1. The IBM SPSS version 20 (IBM Corp., Armonk, NY, USA) was used for the calculations and statistical analysis. ANOVA and Pearson product-moment correlation were calculated as deemed necessary. A p-value <0.05 was considered statistically significant. For further confirmation, a linear correlation coefficient was calculated for the assessment of a possible correlation of the T-DRF decline to the WIT in the operated kidney and to the mass of the resected specimen.
Patients operated with laparoscopic radical nephrectomy
In 15 patients with LRN selected for control group, the mean age was 61±12 years (range: 41–78). The male to female ratio was 11/4, and right to left involved kidney ratio was 12/3. Their mean BMI was 29.7±5.4 (range: 21.6–38.6), and the mean operative time was 153 minutes (range: 100–220). The mean age, BMI and the mean operative time were slightly higher than in the group of LPN.
Results
Twenty eight patients with small renal mass successfully underwent LPN and completed one year follow up according to our protocol. During operation, after hilar unclamping, in six patients we observed mild bleeding from the resected margin, which were resolved within 2-3 minutes by increasing pneumo-peritoneum or application of Surgical. We didn’t have any significant bleeding, necessitate hilar re-occlusion. In our selected patients for statistical evaluations, major intra- or postoperative complication was not observed.
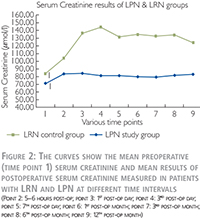
In the LPN group, the mean results of preoperative and postoperative renal function measured by serum creatinine (sCr), estimated glomerular filtration rate (eGFR, CKD-EPI equation) as described by Levey and colleagues (6) and total differential renal function (T-DRF) as well as partial differential renal function (P-DRF) are summarized in Table 2.
Evaluation of the renal function based on serum creatinine
The detailed mean values of these tests are shown in Table 2 for LPN and Figure 2 for both LPN and LRN patients. In Figure 2, the preoperative (time point 1) mean values of serum creatinine as well as the mean values at the eight postoperative check points is shown for both LPN and LRN groups. In LRN group we had elder population (61±12 years vs. 50.5±11.9 years, p-value = 0.008), more male to female ratio (73% vs. 57%) and higher body mass index (BMI) (29.7±5.4 vs. 27.6±4.3, p-value = 0.18). These data may explain the higher baseline (84 µmol/l) serum creatinine in the LRN group. It was a strong increase of serum creatinine level immediately after LRN with a peak on the 3rd post-op day. It declined slightly and remained constantly high (123 µmol/l) until the end of first postoperative year. This reflects ~ 46% rise in Se creatinine level compared to the baseline in LRN patients. In contrary, in the LPN group, the serum creatinine level was only slightly increased from 71 µmol/l to 82 µmol/l (~16% increase) during the first three postoperative days and remained constant until the end of observation.
Evaluation of the renal function based on estimated GFR
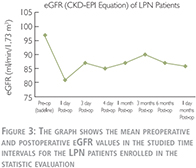
As shown in Table 2 and Figure 3, the mean preoperative or baseline eGFR of patients treated with LPN was 97±17 (range: 55–122) which decreased to 81±21 (range: 44–114) in the 1st postoperative day (p-value = 0.0069). This shows a 16% decline in average eGFR which was the largest postoperative drop within one year of follow up. Conventionally, we call it “transient-state” of kidney function deterioration. In the 3rd postoperative day, we observed a 7% recovery in the average eGFR comparing to the 1st day. Although, due to the large deviations in the values, this tendency toward recovery was statistically insignificant (p-value = 0.3821). From the 3rd postoperative day to end of the study in 12th month, the average eGFR remained roughly the same in both groups. Accordingly, for simplicity, average of all postoperative eGFR mean values after the transient-state (after the 1st day) were calculated and considered as the “steady-state” of renal function deterioration. This was 87 ml/min/1.73 m2 in LPN patients which demonstrates ~10% decrease in renal function comparing to the baseline (p-value = 0.0757).
As shown in Figure 4, the eGFR was lower for patients treated with LRN and showed a decrease during the first three postoperative days and thereafter remained at the same level. By this comparison, the difference in clinical parameter between the two groups as indicated above also should be taken into account.
It is worth to highlight that in the LRN control group, the average decline in the “steady state” of the kidney function was ~ 40% (81.34 declined to 48.36 ml/min/1.73 m2).
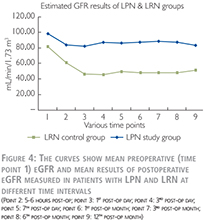
In a simple explanation, we can conclude that, by removal of one whole kidney, nearly 40% of global kidney function declines, and by removal (resection) of one part of a kidney, nearly 10% of the global kidney function decreases. Accordingly, parenchymal volume reduction after partial nephrectomy has a very important impact on outcome of kidney function and this should be certainly distinguished from the impact of the IR injury caused by WI.

Impact of parenchymal volume reduction on the kidney function
As seen in Table 2 and Figure 5, in the LPN study group, the mean preoperative or baseline T-DRF of the operated kidneys was 49% which is decreased to 42% on the 1st postoperative month (p-value = 0.001). This value remained almost the same in the following time points. Repeated ANOVA measurements of all postoperative T-DRF did not show any significant alteration among them (p-value >0.6). Accordingly, average of all postoperative T-DRF which was 42% was considered as the final postoperative result. On the other hand, the mean preoperative P-DRF of the intact pole of the operated kidney was 50% which decreased to 47% on the 1st postoperative month (p-value = 0.0727). Average of all postoperative P-DRF was also 47% without any significant alteration among the time periods (p-value = >0.1).
Linear correlation coefficient was used to compare relationship of the T-DRF decline in the operated kidney to the mass of the resected specimen (Figure 6).
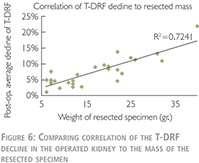
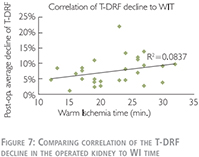
We have also used linear correlation coefficient to compare relationship of the T-DRF decline in the operated kidney to WIT (Figure 8). This showed a much stronger correlation between T-DRF decline and the resected mass comparing to the WI time (R2 = 0.7241 and p0.0837 respectively).
Discussion
In 1950, Benjamin Abeshouse wrote “Few procedures provide the urologist with more satisfaction than those that preserve renal function” (9). While Abeshouse may have practiced urology prior to the availability of the strong data we now possess, his statement rings true to this day. On this principle, NSS has taken a prominent position at the helm of the treatment of renal tumours. Likewise, there has been continual progress toward resecting less and less renal parenchyma to preserve more renal function without sacrificing any of oncological rules.
There are several factors determining the postoperative renal function:
- the preoperative quality of renal function (underlying renal disease, limited glomerular function, etc.);
- the quantity of renal parenchyma remained after operation;
- the warm ischemia time.
The first factor can’t be modified by surgical technique; the second is determined by the anatomical size and location of the tumour. The warm ischemia time is influenced by the experience of the surgeon and the operation technique applied.
We planned a prospective study in order to distinguish the impact of parenchymal loss and effect of warm ischemia on the function of operated kidney. In our study, 99TmTc-DMSA isotope was used which is a static renal agent and allows accurate calculation of DRF (7). Since 99TmTc-DMSA scan provides relative functional percentage of the two kidneys and the contralateral kidney served as a control for comparison after LPN, we selected patients with normal contralateral kidney. Such selections have resulted in a young cohort of patients with mean age of 50.5±11.9 years old in our study. The T-DRF was measured in all isotope studies. Any postoperative decline in T-DRF in the operated kidney was considered as a result of warm ischemia and ischemia-reperfusion injury combined with parenchymal loss. In nearly all postoperative studies, mean decline of T-DRF in the operated kidney was 7%. In order to distinguish the effect of warm ischemia from the parenchymal loss, we introduced the so-called P-DRF in which a region of interest was selected on non-tumorous pole of the involved kidney and it was compared with the same region in the contralateral kidney.
Any postoperative functional decline in this intact pole of the operated kidney was considered to be as a result of warm ischemia and ischemia-reperfusion injury only. In our study, mean postoperative decline in the P-DRF of the operated kidney was only 3% which was found to be statistically insignificant (p-value = 0.0727). In agreement with the previous studies, we believe that within certain time limits of warm ischemia, which was 22±5.3 minutes in our study, the ischemia-reperfusion insult may be negligible or reversible. On the other hand, deliberate parenchymal loss plays a major role in kidney function deterioration.
Warm ischemia and the IR injury to the kidney have been considered for a long time as the main factor related with postoperative renal function deterioration in patients undergoing PN under WI (1, 10–13). Several technically challenging techniques have been introduced for the reduction of WI (14, 15). Nevertheless, the impact of renal parenchymal mass reduction was not distinguished from the effects of WI and IR in the above literature. Parenchymal loss after PN occurs as a result of intentional tumor excision, some normal parenchyma resection and suturing. Thus, the mass or volume of the parenchymal loss should be considered and differentiated from IR injury when evaluating the renal functional outcome after PN.
Few authors have studied the impact of parenchymal volume reduction on renal function. In a large multicenter study cohort Shikanov and colleagues (16) assessed the influence of renal ischemia on long-term global renal function after LPN in patients with 2 functioning kidneys. They retrospectively evaluated eGFR of 401 patients with median tumor size of 25 mm and normal contralateral kidney who underwent LPN with median warm ischemia time of 29 minutes. Changes in early postoperative and nearly one year follow-up eGFR were –16% and –11%, respectively. This result is nearly identical to our findings. The percent change in last eGFR was worse in patients with larger tumors suggesting that excising a larger lesion would result in more loss of renal parenchyma. Mir and colleagues (17) also reported that the preservation of the total renal function after PN ranged between 88% and 91% which reflects approximately 10% loss of the renal function (similar to our finding). Mir et al also showed that a higher reduction of the eGFR in patients with larger tumors which could be attributed to the excision of a larger lesions and the consequent greater loss of renal parenchyma. This was documented also by Sharma and colleagues (18) in 21 patients with solitary kidney who underwent partial nephrectomy due to small renal mass. Volume of the kidney was measured preoperatively and postoperatively by CT scan and software with an automated segmentation algorithm. An average of 15% parenchymal volume loss and 19.7% deterioration in kidney function were documented in mid-term postoperative period. They concluded that the percent of renal parenchymal volume loss was correlated with the percent loss in eGFR. It was shown that volume loss had a more direct, predictable effect on ultimate eGFR than ischemia time. A similar result was concluded by Simmons and colleagues (3) and also Song and colleagues (19). In the later study, diethylene-tetramine penta-acetic acid (99mTc-DTPA) was used for accurately estimating renal clearance and measuring eGFR by the kidney. They measured changes in individual renal function and investigated factors determining the degree of functional reduction in 65 patients who underwent LPN and 52 patients with open partial nephrectomy. In the entire cohort, renal volume reduction was the most significant, independent prognosticator for eGFR reduction after partial nephrectomy. Mir and colleagues (20) evaluated the role of volume preservation by introducing computed tomography volumetric analysis using free-hand scripting. The eGFR was measured by MAG3 in 92 patients who underwent partial nephrectomy. The authors revealed that the ultimate renal function after partial nephrectomy was primarily driven by parenchymal preservation with ischemia playing a secondary role as long as it was within a limited time period.
Current literature has not concluded to the most important factor for the renal function decline after PN and the contribution of WI to the postoperative renal function has not been well documented (17). Some investigators advocated that the parenchymal mass preservation was stronger correlated to the functional recovery in comparison to the WI (4, 19).
The current prospective study aimed in distinguishing the impact of parenchymal loss from the WI effect on the operated kidney. The 99mTc-DMSA isotope was used for the purpose due to the fact that it allows accurate calculation of DRF (7). The latter parameter was measured preoperatively and in different postoperative intervals in 28 patients with solitary small polar tumours. Since 99mTc-DMSA scan provided relative functional percentage of the two kidneys, the contralateral kidney served as the control for the comparison after LPN. Consequently, only patients with normal contralateral kidney were selected and a young patient population with mean age of 50.5±11.9 years was eventually included in the study. Any postoperative decline in the T-DRF of the operated kidney was considered as a result of WI and WI combined with parenchymal loss. In nearly all postoperative studies, a mean decline of 7% in the T-DRF was noted. In an attempt to distinguish the effect of WI from the parenchymal loss, the P-DRF was introduced. A ROI was selected on the non-tumorous pole of the involved kidney and was compared with the same ROI on the contralateral kidney. Any interference of the excision area to the ROI was prevented by including only patients with tumour mass of £4 cm in diameter located on either upper or lower pole of the kidney. Any postoperative functional decline in this intact pole of the operated kidney was considered to be as a result of WI only. The mean postoperative decline in the P-DRF of the operated kidney was only 3% which was found to be statistically insignificant (p-value = 0.072). In agreement with the previous studies (21), it could be suggested that WI may result in negligible or reversible renal damage within certain time limits of WIT such as a mean WIT of the current study. In addition, the parenchymal loss seemed to play a more important role in kidney function deterioration than WI. Considering the above, it could be advocated that the LPN surgical technique could probably focus on the precise tumour excision and suturing rather than to the minimization of WIT. Nevertheless, additional studies are necessary for the confirmation of the above hypothesis.
Limitations of this study include the reliance on DRF and the use of the non-operated kidney as a stable reference unit before and after the surgery. Any postoperative compensatory hypertrophy of the contralateral kidney may result in a false outcome of DRF. Takagi et al. (22) showed that the compensatory hypertrophy of the contralateral kidney after PN remained rather limited and less than 2.3% in most cases. They concluded that the larger the excised volume of the kidney, the more hypertrophy of the contralateral kidney was expected. The median tumour diameter of the latter study was 3.5 cm and probably resulted in higher volume loss in comparison to our series (median of 2.6 cm). Hence, we assume that the compensatory hypertrophy may have been negligible in our study.
Another limitation of our study was the lack of stratification of the results according to the length of the WIT or the tumour size. The parenchymal volume was never measured and the current study could not provide information regarding the pre-and postoperative changes in the volume of the renal parenchyma. Nevertheless, the changes in the contour of the operated kidney may influence measurements of the renal volume and the selection of ROIs out of the excision field for measurements probably allowed for more reliable results. Moreover, the use of CT scans for the evaluation of renal volume would expose the patient in additional radiation without providing evidence that would significantly influence the results of the study.
Conclusion
In LPN, the parenchymal loss caused by the resection of the tumor and the suturing of the surrounding normal tissues resulted in kidney function deterioration which should be distinguished from WI effects. An average WIT of 22 minutes for a mean tumor diameter of 2.6 cm resulted in a 7% kidney function decline. 4% could be attributed to the parenchymal loss and 3% to WI.
The ultimate renal function after partial nephrectomy is primarily driven by parenchymal preservation with ischemia playing a secondary role as long as it is within a limited time period. One of the major implications of our study is that creating a bloodless filed by clamping the renal pedicle within certain time limits, and consequently precision of excision and renorrhaphy, should be a primary objective during any partial nephrectomy. This may result in more kidney function preservation than putting all efforts to decrease WI time to zero while accepting all potential complications.
References
1. Becker F, Van Poppel H, Hakenberg OW, Stief C, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 2009; 56: 625–34. https://doi.org/10.1016/j.eururo.2009.07.016
2. Simmons MN, Schreiber MJ, Gill IS. Surgical renal ischemia: a contemporary overview. J Urol 2008; 180: 19–30. https://doi.org/10.1016/j.juro.2008.03.022
3. Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, Campbell SC. Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J Urol 2012; 187: 1667–1673. https://doi.org/ 10.1016/j.juro.2012.02.1218 https://doi.org/10.1016/j.juro.2011.12.068
4. Song C, Park S, Jeong IG, Hong JH, Park HK, Kim C-S, Ahn H. Followup of unilateral renal function after laparoscopic partial nephrectomy. J Urol 2011; 186: 53–58. https://doi.org/10.1016/j.juro.2011.03.021
5. Spaliviero M, Gill IS. Laparoscopic partial nephrectomy. BJU international 2007; 99: 1313–1328. https://doi.org/10.1111/j.1464-410X.2007.06809.x
6. Levey AS, Stevens LA, Schmid CH, Zhang YL, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009; 150: 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
7. Kibar M, Yapar Z, Noyan A, et al. Technetium-99m-N,N-ethylenedicysteine and Tc-99m DMSA scintigraphy in the evaluation of renal parenchymal abnormalities in children. Ann Nucl Med 2003; 17: 219–225. https://doi.org/10.1007/BF02990025
8. Rutland MD. Origin of the Patlak-Rutland plot. Nucl Med Commun 1996; 17: 441. https://doi.org/10.1097/00006231-199605000-00015
9. Abeshouse BS, Lerman S. Partial nephrectomy versus pyelolithotomy and nephrolithotomy in the treatment of localized calculous disease of the kidney, with a report of 17 partial nephrectomies. Int Abstr Surg 1950; 91(3): 209–40.
10. Thompson RH, Lane BR, Lohse CM, et al. Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur Urol 2010; 58: 31–336. https://doi.org/10.1016/j.eururo.2010.05.048
11. Choi JD, Park JW, Lee SY, et al. Does prolonged warm ischemia after partial nephrectomy under pneumoperitoneum cause irreversible damage to the affected kidney? J Urol 2012; 187: 802–806. https://doi.org/10.1016/j.juro.2011.10.140
12. Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 2013; 24: 506–517. https://doi.org/10.1681/ASN.2012080786
13. Porpiglia F, Renard J, Billia M, et al. Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol 2007; 52: 1170–1178. https://doi.org/10.1016/j.eururo.2007.04.024
14. Gill IS, Patil MB, Abreu AL, et al. Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol 2012; 187: 807–814. https://doi.org/10.1016/j.juro.2011.10.146
15. Nguyen MM, Gill IS. Halving ischemia time during laparoscopic partial nephrectomy. J Urol 2008; 179: 627–632. https://doi.org/10.1016/j.juro.2007.09.086
16. Shikanov S, Lifshitz D, Chan AA, et al. Impact of ischemia on renal function after laparoscopic partial nephrectomy: a multicenter study. J Urol 2010; 183: 1714–1718. https://doi.org/10.1016/j.juro.2010.01.007
17. Mir MC, Ercole C, Takagi T, Zhang Z, Velet L, et al. Decline in renal function after partial nephrectomy: etiology and prevention. J Urol 2015; 193: 1889–1898. https://doi.org/10.1016/j.juro.2015.01.093
18. Sharma N, O’Hara J, Novick AC, et al. Correlation between loss of renal function and loss of renal volume after partial nephrectomy for tumor in a solitary kidney. J Urol 2008; 179: 1284–1288. https://doi.org/10.1016/j.juro.2007.11.071
19. Song C, Bang JK, Park HK, et al. Factors influencing renal function reduction after partial nephrectomy. J Urol 2009; 181: 48–53. https://doi.org/10.1016/j.juro.2008.09.030
20. Mir MC, Campbell RA, Sharma N, et al. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology 2013; 82: 263–268. https://doi.org/10.1016/j.urology.2013.03.068
21. Zargar H, Porpiglia F, Porter J, Quarto G, Perdona S, et al. Achievement of trifecta in minimally invasive partial nephrectomy correlates with functional preservation of operated kidney: a multi-institutional assessment using MAG3 renal scan. World J Urol 2016 Jul; 34(7): 925–31. https://doi.org/10.1007/s00345-015-1726-x
22. Takagi T, Mir MC, Sharma N, et al. Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol 2014; 192: 1612–1619. https://doi.org/10.1016/j.juro.2014.06.018
Leave a Reply
You must be logged in to post a comment.